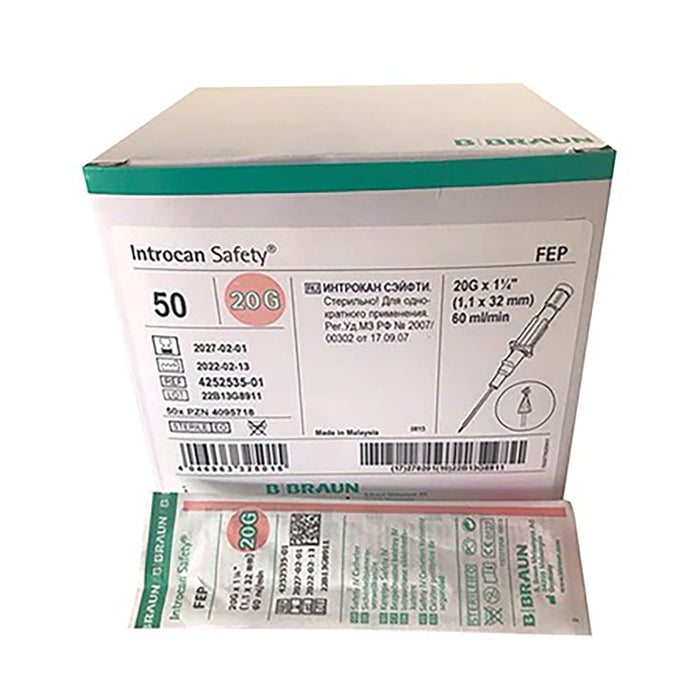
Introcan Fep 20g x 32mm Pink 1 Unit
Introcan FEP 20G x 32mm Pink – Peripheral IV Cannula (1 Unit)
Introcan FEP 20G x 32mm is a sterile, single-use peripheral IV cannula designed to provide safe, reliable venous access for infusion, medication administration and blood sampling. The pink 20G colour coding and smooth fluorinated ethylene propylene (FEP) catheter help ensure atraumatic insertion, reduced friction and improved patient comfort during short- to medium-term IV therapy.
Key Benefits
- Sterile, single-use peripheral IV cannula for safe venous access and infusion therapy.
- 20G x 32mm pink hub – standard colour coding for quick size identification.
- FEP catheter material offers low friction and kink resistance for easier insertion and reliable flow.
- Sharp stainless steel introducer needle facilitates smooth venipuncture and atraumatic entry.
- Ideal for medication administration, fluid therapy and blood sampling in hospitals, clinics and outpatient settings.
- Latex-free construction to reduce risk of allergic reaction.
Ingredients and Materials
Manufactured from biocompatible materials suitable for intravenous use: fluorinated ethylene propylene (FEP) catheter tubing, stainless steel introducer needle, and a durable polymer hub (polypropylene or polycarbonate). Sterile, single-use, latex-free components. Colour-coded pink hub identifies 20G size for fast clinical reference.
How to Use
- Verify product integrity and expiry; confirm single-use sterile packaging is intact.
- Follow local aseptic technique and venipuncture protocol. Prepare site and select appropriate vein.
- Insert needle and catheter at the recommended angle; advance catheter into vein, then withdraw the needle.
- Secure cannula with dressing, flush with saline to verify patency, and label with date/time if required.
- Monitor site for signs of infiltration, phlebitis or infection; replace or remove per local policy.
- Dispose of needle and cannula components as regulated medical/sharps waste after single use.
Who It’s For
Designed for healthcare professionals who perform peripheral intravenous access: nurses, phlebotomists, paramedics and clinicians in hospitals, surgical units, emergency departments, infusion centres, primary care clinics and first aid settings. Suitable for adult and adolescent patients requiring IV therapy where a 20G catheter is clinically indicated.
Caution & Storage
For single use only. Do not use if packaging is damaged or if sterility is compromised. Follow local clinical protocols and aseptic technique during insertion. Observe the insertion site regularly for infection, thrombosis, infiltration or extravasation. Not for use in patients with known contraindications to peripheral IV access at the chosen site.
Store in a cool, dry place away from direct sunlight. Dispose of all components as clinical/sharps waste according to local regulations. Seek immediate medical attention if complications (pain, swelling, redness, fever) occur at the cannulation site.
Keywords: Introcan FEP, 20G, 32mm, IV cannula, peripheral IV cannula, venous access, infusion, medication administration, blood sampling, sterile single-use, latex-free.
